(J=W*s ;julios igual vatios por segundo)
1 eV = 1,620 10^-19 J
1 J = 6,1728 10^18 eV
1 julio o joule (J) es igual a 6.241509⋅1018 electron-volts (eV) *sacado de internet.
Sobre electro-imanes.
Sabiendo que los iones de protio (isótopo de hidrógeno) y otros iones en el magnetismo o dimensión magnética, generan la energía de la electricidad circulando por el campo magnético.(Teoria del Todo)
Se puede explicar el funcionamiento por ejemplo de los electro-imanes.
En los electro-imanes con núcleo (metal), este núcleo multiplica el campo magnético generado por la bobina al pasar los iones por el (el núcleo de metal): pues alinea el campo magnético de los átomos del metal, (a parte de generar calor por la desintegración de los iones) .
El efecto es como apilar imanes.
Sabiendo esto:
Un electro imán debería ser capaz de absorber iones y generar calor al chocar algunos iones con los átomos del metal del núcleo.
La bobina no debería calentarse en principio pues el campo magnético pasaría por el núcleo, debería generar energía; no de la" corriente" sino de los iones absorbidos no sería fusión exactamente. Todos los electro-imanes se calientan y es por esto habría que ver que les pasa si los expones a diferentes dosis de radiación si pueden proteger de esta como la tierra lo hace con su campo magnético del viento solar.
Energía generada electro-imán.
Fundir metal por inducción.
(Pese a que no tiene núcleo de hierro que aumente el efecto magnético de la bobina un horno de fundición de metales me parece interesante pues tiene una bobina que genera un campo magnético.)
De la misma manera que funcionan las cocinas eléctricas; por inducción también hay maquinas que funden metal, y revisando maquinaria industrial realicé unos cálculos.
"Como se mencionó anteriormente, el consumo de energía estándar para la fundición de chatarra ligera de aluminio requiere 600 a 625 kWh/tonelada (considere un promedio de 612,5 kWh). Eso significa que una tonelada de chatarra ligera de aluminio requiere de 600 a 625 kWh".
Cálculo:
1 W (vatio) = 1 J (julio) * s (segundo)
1 kW⋅h = 3,6 MJ
625 kWh → 2.250 MJ (mega julios) para fundir 1000 Kg
2.250.000 J → 1 Kg
Vamos a calcular que energía tiene el aluminio fundido de 660 ºC respecto al aluminio solido de 0ºC y usaremos ese valor para orientarnos.
“Energía para fundir un kilo 900 julio/kilogramo”
“Calor específico: El calor específico de una sustancia se define como la cantidad de julios (o calorías) necesaria para cambiar la temperatura de exactamente 1 g de una sustancia por exactamente 1 C. “
“La capacidad calorífica es la cantidad de calor absorbida (emitido) todo el cuerpo en proceso de calentamiento (enfriamiento) por 1 Kelvin. “
“En termodinámica, la entalpía de fusión de una sustancia, también conocida como calor (latente) de fusión, es el cambio en su entalpía resultante de proporcionar energía, típicamente calor, a una cantidad específica de la sustancia para cambiar su estado de un sólido a un líquido, a presión constante. Por ejemplo, al derretir 1 kg de hielo (a 0 °C bajo un amplio rango de presiones), se absorben 333,55 kJ de energía sin cambio de temperatura. El calor de solidificación (cuando una sustancia cambia de líquido a sólido) es igual y opuesto.”
datos (1):
Masa
atómica aluminio: 26,9815386 u gramos por mol (sale de la tabla periódica)
Calor especifico del aluminio: 0.9 J/g K.
Punto de fusión Aluminio: 933,47 K (660 °C)
Entalpía de fusión aluminio: 10,71 kJ/mol
datos(2):
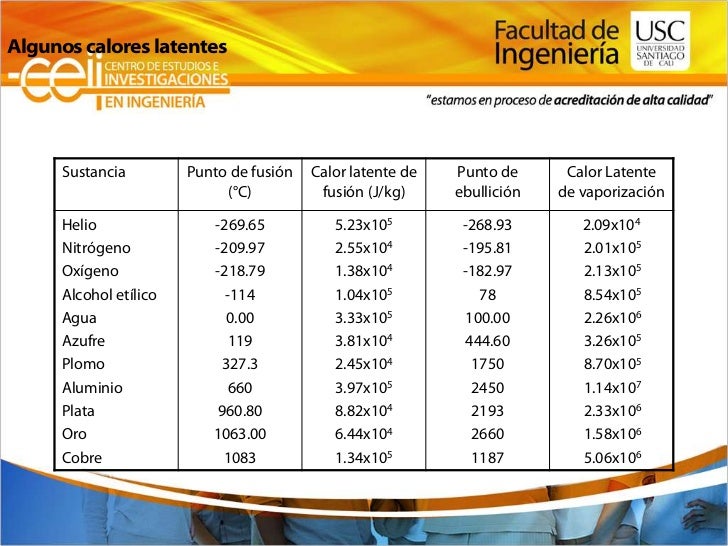
Sustancia |
T fusión ºC |
Lf ·103 (J/kg) |
T ebullición ºC |
Lv ·103 (J/kg) |
|---|---|---|---|---|
Hielo (agua) |
0 |
334 |
100 |
2260 |
Alcohol etílico |
-114 |
105 |
78.3 |
846 |
Acetona |
-94.3 |
96 |
56.2 |
524 |
Benceno |
5.5 |
127 |
80.2 |
396 |
Aluminio |
658.7 |
322-394 |
2300 |
9220 |
Estaño |
231.9 |
59 |
2270 |
3020 |
Hierro |
1530 |
293 |
3050 |
6300 |
Cobre |
1083 |
214 |
2360 |
5410 |
Mercurio |
-38.9 |
11.73 |
356.7 |
285 |
Plomo |
327.3 |
22.5 |
1750 |
880 |
Potasio |
64 |
60.8 |
760 |
2080 |
Sodio |
98 |
113 |
883 |
4220 |
Fuente: Koshkin N. I., Shirkévich M. G.. Manual de Física elemental, Edt. Mir (1975) págs. 74-75.
Total:
Total:
Earlier we calculated 396.93 kJ to go from
solid to liquid.
594 kJ+ 396.93 kJ
990.93
kJ
Using data(2)
0.9 J/g K * 658.7 K =0.9 kJ/kg
K * 658.7 K= 594 kJ to raise the temperature by 658.7 K to one
kilo.
Total:
592.83 kJ
592.83 kJ
+322 kJ
914.83 kJ
Energy of a kilo of aluminum
already melted at 660°C compared to solid aluminum at 0°C
914.83
kJ is needed for a kilo of aluminum to melt from 0ºC to 660ºC at
least. For that is the energy it possesses at that temperature.
The
machinery has needed
2250 kJ
So it does not
generate energy in the form of heat (it was a scrap melting
machine).
Or so it seems.
It happens that
aluminum is not ferromagnetic and does not multiply the effect of the
electromagnet, and does not affect because the protons or ions have
been attracted by electricity even so the heat has been generated by
the disintegration of these as we have seen before.
Ferromagnetic
materials would be:
Iron (Fe)
Cobalt
(Co)
Nickel (Ni)
Steel
alloys
etc.
With ferromagnetic materials,
electro-magnets can be created by the effect I described above.
"An
object is considered ferromagnetic if a magnet sticks to it."
"Is
aluminum ferromagnetic?
Aluminum is one of the
paramagnetic metals, which means that magnets only weakly attract it.
So weakly that you might think that they have no influence on
aluminum. However, the video presented to you here demonstrates that
magnets do have an effect on aluminum."
The information I have:
Energy consumption to
melt 1 ton metals in one hour:
For melting cast iron
it takes 550-575 kWh/ton.
For SG Iron melting it requires
550-600 kWh/ton.
For Stainless Steel/Steel melting 600-650
kWh/ton is required
For Aluminum light scrap melting
600-625 kWh/ton is required.
500-575 kWh/ton is required
for melting Aluminum chopped scrap.
For steel melting 625
kWh/ton is required.
Weight per cubic meter metals:
Steel 7850 kg/m³
?
Aluminum 2700 kg/m³ 26.9815
Bronze with tin
8900 kg/m³ ?
Bronze with lead 8700 kg/m³ ?
Copper
8960 kg/m³ 63.5460
Iron 7300 kg/m³ 55.8450
Brass
8560 kg/m³ ?
Aluminio |
2700 kg/m³ |
26.9815 g/mol |
600-625 kWh/ton |
Cobre |
8960 kg/m³ |
63.5460 |
? |
Hierro |
7300 kg/m³ |
55.8450 |
550-575 kWh/ton |
Acero |
7850 kg/m³ |
? |
625 kWh/ton |
Aluminio (chatarra troceada) |
? |
? |
500-575 kWh/ton |
Aluminum with 2700 kg/m³ is the lightest but requires 600-625 kWh/tonne.
Iron with a density of 7300 kg/m³ per cubic meter is 2.7 times denser than aluminum.
For the same weight:
For the melting of light Aluminum scrap 600-625 kWh/ton is required.
500-575 kWh/ton for the melting of aluminum scrap
Cast iron melting requires 550-575 kWh/tonne
Aluminum 0.0269815 Kg/mol
Iron 0.0558450 Kg/mol
0.0000269815 Ton/mol 37,062.4316 mole of Aluminum per ton of Aluminum.
0.0000558450 Ton/mol 17,906.7060 mole of Iron per ton of Iron.
There are 2.0697 times as many moles of aluminum as of iron in a ton.
And the mole of iron is 2.0697 times heavier than the mole of aluminum.
The energy of melting cast iron for the machine 575 kWh
and I calculate the joules:
575 kWh
575 000 W * 3600 s
2,070,000,000,000 J/s (W)
2.250 kJ → 1 Kg Aluminium.
83.390,4711 kJ * 37,0624 mol → 0,4444 J/mol
2,070 kJ → 1 Kg Iron.
37.066,88142 kJ * 17,9067 mol → 0,4830 J/mol
Despite the difference in density and weight per cubic meter the performance of the machines is similar in terms of heat in joules to melt one mole.
I continue with the checks:
Stationary Acutrak®
Power Density
150 Kilowatts per Ton
Power Requirements
167 KVA, 460/480 Hz, 50/60 Hz
https://www.inductotherm.com/products/acutrak-direct-electric-heat-deh-system/
for 167 KVA
approx 0.9 power factor
150300 W (150 kW per Ton)
for one ton 1 T or 1000Kg
(150 kWh/T)=
in one hour 60 minutes * 60 seconds
150300*(360)
54.108.000 J
I will calculate the results in electron volts as well:
"The electron volt is a unit of energy that represents the energy variation experienced by an electron when moving from a point of potential Va to a point of potential Vb when the difference Vba = Vb-Va = 1 V, that is, when the potential difference of the electric field is 1 volt."
3.3771558755e+26 eV
914,830,000 J minimum to melt 1000 kg of aluminum from 0 to full melting.
5.7099 10^27 eV
i.e:
But with initial data (not catalog data):
625 Kilowatt hours would consume to melt 1000 kilos of aluminum
2.250.000.000.000 J to melt 1000 kg
1.4043 10^28 eV
Something strange is going on here...
I look at industrial machinery below.
https://www.induction-furnace.com/products/aluminum-melting-furnace/
Aluminum Melting Furnace
Miro maquinaria industrial a continuación.
https://www.induction-furnace.com/products/aluminum-melting-furnace/
Aluminum Melting Furnace
Model |
Power Control Cabinet |
Furnace Body |
Associated Transformer(KVA) |
|||||||
Power |
Voltage |
MF Voltage |
Cabinet Size(mm) |
Frequency |
Weight |
Furnace body Size(mm) |
Induction Coil Inner Diameter |
Weight |
||
0.1T |
80kw |
3×380v |
750v |
1000×800×1700 |
2500 |
0.5T |
Φ730 |
Φ500 |
0.7T |
125 |
0.25T |
180kw |
3×380v |
750v |
1300×850×2000 |
2000 |
0.8T |
Φ1100 |
Φ580 |
1T |
250 |
0.35T |
200kw |
3×380v |
750v |
1300×850×2000 |
1500 |
0.8T |
Φ1100 |
Φ700 |
1.2T |
400 |
0.5T |
400kw |
3×380v |
1500v |
1300×850×2000 |
1500 |
1T |
Φ1250 |
Φ760 |
1.5T |
500 |
0.75T |
600kw |
3×380v |
1500v |
1300×850×2000 |
1000 |
1T |
Φ1350 |
Φ840 |
1.6T |
630 |
1T |
700kw |
3×660v |
2400v |
1300×850×2000 |
800 |
1.2T |
Φ1400 |
Φ960 |
1.8T |
900 |
1.5T |
8000kw |
3×660v |
2500v |
1300×850×2000 |
600 |
1.2T |
Φ1700 |
Φ1150 |
2.5T |
1250 |
2T |
1000kw |
3×660v |
2500v |
1300×850×2000 |
600 |
1.6T |
Φ1700 |
Φ1350 |
3T |
1600 |
3T |
1500KW |
3×950v |
3200v |
2400×1000×2000 |
500 |
1.6T |
Steel shell |
Φ1450 |
12T |
2500 |
5T |
2000KW |
6×950v |
3400V |
2400×1000×2000 |
500 |
2.5T |
Steel shell |
Φ1800 |
19T |
3300 |
Product Introduction
The heating furnace is mainly used for heating of metal materials before forging, extrusion, hot rolling, shearing, and heat treatment of metal materials, such as quenching, tempering, tempering and the like. Heating temperature is between 200-1200 degrees.
Pre-forging heating: applied to gear, ring gear, bearing, shackle, rigging.
Online heating: Pipe anti-corrosion coating, long steel rod heating, steel (wire) tube online quenching and tempering.
Local heating: U-bolt bending, thermal assembly of the drum,Steel pipe elbow etc.
Product Features
The induction coil is insulated by two insulation treatment, high temperature insulating paint and fiberglass ribbon winding.
The top of the furnace body is designed with sliding cover, which is convenient for maintenance and inspection.
Equipment installation and operation is simple.
Equipment covers a small area
Stable and reliable heating temperature
Power consumption280kwh/ton
Technical Parameter
Technical Parameter
Model |
Power Input(3-Phase 4-Wire 50/60Hz) |
Power Output |
Capacity(Kg/h) |
|||||||
Power |
Voltage |
Current |
DC Current |
DC Voltage |
MF Voltage |
MF Frequency |
Steel |
Copper |
Aluminum |
|
KGPS-50 |
50KW |
380V |
80A |
100A |
500V |
750V |
1-8KHZ |
125 |
282 |
234 |
KGPS-50 |
50KW |
380V |
80A |
100A |
500V |
750V |
1-8KHZ |
125 |
282 |
234 |
KGPS-750 |
750KW |
380V 660V |
1200A 680A |
1500A 850A |
500V 880V |
750V 1300V |
0.2-6KHZ |
1875 |
4230 |
3510 |
Working Temperature: Steel 1250℃, Copper 900℃,Aluminum 500℃ |
||||||||||
Power Consumption: Steel 350-550KWH/T, Copper 150-300KWH/T, Aluminum 180-400KWH/T |
||||||||||
I will calculate the maximum consumption: 400kWh per ton.
400kWh
per 1000 Kg
400000Wh per 1000 Kg
1,440,000,000
W(J) in one hour.
1,440,000,000 joules(W) per
kilo.
Electron-volt
8.9877 10^24 eV per kilo.
potencia de entrada: 50KW 380V 80A
potencia de salida: 100A 500V (DC)
Capacidad: 234Kg/h
We calculate:
50000 W
18,000,000 J in one hour
(234 Kg)
76,923,0769 J one kilo
76.923.076,923
J 1000 Kg (1 T)
76.923.076,923 J/(1 T) / 18.000.000 J in
one hour (234 Kg)
4.2735 hours to melt 1000 Kg (1
T)
4.8011610333 10^26 ( one joule or joule (J) is equal to
6.241509⋅1018 electron-volts (eV))
50000 W * 4.2735
h
213.675 kWh/T
that system would need
213,675 kWh/h to melt 1000 kg (1T) in one hour then
is
within the range: Aluminum "180-400KWH/T" and what they
suggest "Power consumption 280 kwh/ton".
compare
with previous system: (Acutrak® Melting Systems aluminum melting
machine from www.inductotherm.com (150 Kilowatts per Ton per Hour)).
167 kVA now at 380
=253.73 A
=96.417,4
W
34,710,264 J an hour (almost double)
and
if they had the same efficiency it would have melted:
34,710,264 / 76,923.0769 J one kilo.
451.2334
kg in one hour
2.21614 h to melt 1000 Kg(1 T)
or
76.923.082,3782 J to melt 1000 Kg (1 T)
54.108.000
J to melt 1000 Kg (this is the data that gives 150 kW/T) so the first
one was more efficient but they are very similar in performance.
I
recalculate the minimum energy to melt aluminum with another method
and get out of doubt.
Formula used:
"Thermal energy = Specific gravity of
material*Volume of molten metal*(Specific heat capacity*(Base metal
melting temperature-Ambient temperature)+Latent heat of fusion).
Q = (SG*V*(c*(Tm-ta)+ΔHf)*4.2)/(1-R)
This
formula uses 8 Variables.
Variables utilizadas:
Thermal energy - (Measured in Joule) - Thermal energy is the total
amount of heat required.
Material specific gravity
- Material specific gravity is a dimensionless unit defined as the
ratio of the density of the material to the density of water at a
specific temperature.
Molten Metal Volume -
(Measured in Cubic Meter) - Molten metal volume is defined as the
volume of material removed during the LBM process.
Specific
heat capacity - (Measured in joule/kilogram/K) - Specific heat
capacity is the heat required to raise the temperature of the unit
mass of a given substance by a given amount.
Base
metal melting temperature - (Measured in Kelvin) - The melting
temperature of the base metal is the temperature at which its phase
changes from liquid to solid.
Ambient temperature - (Measured in
Kelvin) - The ambient temperature is the temperature of the
surroundings.
Latent heat of fusion - (Measured in
joule/meter³) - The latent heat of fusion or enthalpy of
solidification is the heat released during solidification. Enter the
magnitude only. It is taken negative by default."
2.7 Specific gravity of the material [SG] times density water with
respect to water
1 Volume of molten metal [V] cubic
meter
8.96 Specific heat capacity [c] kilojoule per
kilogram
933.47 K Melting temperature of the base metal
[Tm] kelvin
0°C + 273.15
= 273.15 K Ambient
temperature [ta] kelvin
3.97*10^5 (J per kilo) *2700 (kg
per meter³ ) = 1,071,900,000 J Latent heat of fusion [ΔHf] joule
meter3
0.9 Reflectivity of the material [R]"
I have adjusted the formula for the data I have:
Q =
(SG*V*c*(Tm-ta))+ΔHf
(2,700 kg × 1 m³ ×
8.96 kJ/kg × (933.47 ºK - 273.15 ºK)) + 1,071,900 kJ
(2,700
kg × 5,916.4672 kJ) + 1,071,900 kJ
1.597.446,44 kJ +
1.071.900 kJ
2.669.346,44 kJ for 2700 kg
988,6468
kJ for 1 kg
988,646.829 kJ for 1000 kg
6.1706
10^27 eV for 1000 kg
I compare results for melting 1000 kg of
aluminum by induction.
Calculated values for reference use:
914.830 kJ energy as
minimum to melt 1000 kg of aluminum.(from periodic table data and
constants).
5.7099 10^27 eV minimum energy to melt 1000 kg
of aluminum (from periodic table data and constants).
988.646,829
kJ for 1000 kg of aluminum (minimum according to aluminum values and
physics formulas).
6.1706 10^27 eV for 1000 kg of aluminum
(minimum according to aluminum values and physics formulas).
Values
calculated from manufacturer's data and available on the
internet:
2,250,000 kJ per 1000 kg uses the machinery that
I looked at the beginning of the article; as they say 625 kW 1h 1000
kg (as expected by generic data).
1.4043 10^28 eV per 1000
kg uses the machinery I looked at at the beginning of the article;
according to 625 kW 1h 1000 kg (according to expectations by
data).
1,440,000 kJ per 1000 kg (based on the industrial
furnaces for aluminum smelting www.induction-furnace.com that I
mentioned before (maximum energy consumption)).
8.9877
10^27 eV per 1000 kg (based on the industrial furnaces for aluminum
smelting www.induction-furnace.com mentioned above (maximum energy
consumption)).
76,923.0769 kJ per 1000 kg (based on the
industrial furnaces for aluminum smelting www.induction-furnace.com
mentioned above (for the KGPS-50 model)).
4.8011 10^26 eV
per 1000 kg (based on the industrial furnaces for aluminum smelting
www.induction-furnace.com that I have mentioned above (for the
KGPS-50 model)).
54,108,000 kJ 1000 kg (Acutrak® Melting
Systems aluminum melting machine from www.inductotherm.com (150
Kilowatts per Ton per Hour)).
3.3771 10^26 eV 1000 kg
(Acutrak® Melting Systems aluminum melting machine from
www.inductotherm.com (150 Kilowatts per Ton per
Hour)).
Conclusion
Commercial induction machines that
melt aluminum do so with less energy than expected.





No hay comentarios:
Publicar un comentario